Introduction: PEG rhG CSF has been widely used in the prevention and treatment of bone marrow suppression after chemotherapy for a variety of tumors, but whether it can be used for lymphoma and Multiple myeloma autologous hematopoietic stem cell mobilization has no relevant clinical evidence. Therefore we carried out this randomized controlled, multicenter clinical study to evaluate the efficiency and safety of PEG-rhG-CSF in the mobilization of autologous hematopoietic stem cells for lymphoma and Multiple myeloma.
Methods:This experiment selected an appropriate mobilization based on the patient's willingness and physical condition of the subjects. Chemotherapy mobilized patients underwent bone marrow mobilization when white blood cells reached their lowest point and began to rebound. The experimental group received a fixed dose of 12mg of polyethylene glycol recombinant human granulocyte stimulating factor injection (PEG-rhG-CSF) for mobilization; the control group received recombinant human granulocyte stimulating factor (rhG-CSF) 5-10μg/kg daily. Collect blood and bone marrow samples from patients daily and test white blood cells and CD34+cells every day. Record CD34+cells proportion until its qantity reached 2×10 6 proportion. The interval from mobilization to collection, the time for hematopoietic reconstruction after transplantation, and complications were also collected.
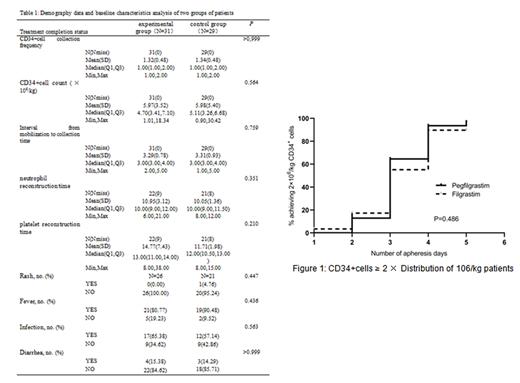
Results : A total of 60 patients were included in this study, including 31 in the experimental group and 29 in the control group. There were no statistically significant differences in gender, age, disease classification, and pre harvest CD34+levels between the two groups of patients. The CD34+cells collected from both the experimental and control groups reached ≥ 2.0×10 6/kg and there was no statistically significant difference (Figure 1). There was no statistical difference between the experimental group and the control group in terms of CD34+cell collection frequency, CD34+cell count, interval from mobilization to collection time, neutrophil reconstruction time, and platelet reconstruction time. In terms of related side effects there was no statistically significant difference in the incidence of rash, fever, infection, and diarrhea between the two groups of patients. The incidence of bone pain in all patients was 15% (9/60), with 8 cases being mild and only 1 case being moderate. There was no significant statistical difference in the incidence of bone pain between the experimental group and the control group (Table 1).
Conclusion : PEG-rhG-CSF is safe and effective for autologous hematopoietic stem cell mobilization in lymphoma and Multiple myeloma, and its efficacy is consistent with that of rhG CSF. However, the subcutaneous injection of rhG CSF is less frequent, which is easier for patients to accept.
[Key words] PEG rhG CSF; matopoietic stem cell mobilization;ymphoma and Multiple myeloma; inical study
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal